Program Management at Inotiv:
Your Partner in Success
With proactive oversight, cross-functional collaboration, and a commitment to your success, we help you navigate drug development with clarity and confidence.

Accelerate Your Development Journey
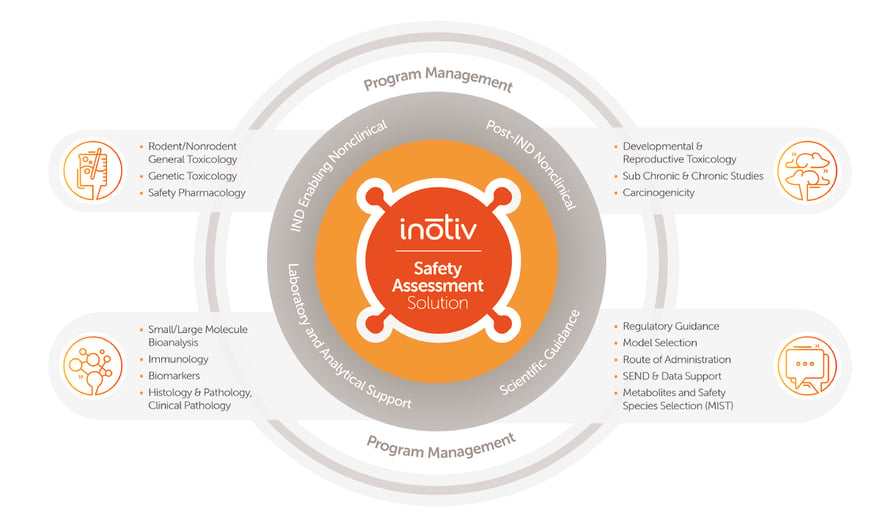
Inotiv is your trusted partner in bringing life-changing products to market. Our integrated discovery-to-development services, coupled with expert Program Management solution, ensure your projects are executed flawlessly, on time, and within budget from award to delivery. Our Program Management team brings a solution to our clients that focuses on TLC and advocacy for their programs.
- Timing – management of timelines and deliverables
- Logistics – management of materials and information
- Communication – collaborative communication plan to meet client’s requirements
- Advocacy – resolving issues and/or confusion throughout the program lifecycle
Why Choose Inotiv’s Program Management Solution?
Our dedicated Program Management team is committed to your success. We offer:
- Single Point of Contact: Enjoy seamless communication and personalized attention.
- Strategic Oversight: We keep your project on track, delivering results when you need them.
- Cross-Functional Collaboration: Our team ensures efficient coordination across disciplines and locations.
- Proactive Problem-Solving: We anticipate challenges and develop solutions to keep your program moving forward.
Our Commitment to You
At Inotiv, we believe in building strong partnerships based on trust, transparency, and exceptional service. Our Program Managers will:
- Understand Your Goals: We take the time to learn your objectives and tailor our approach accordingly.
- Build a Strong Team: We assemble the right experts to support your project.
- Keep You Informed: Regular updates and open communication are essential to your success.
- Deliver Results: We are committed to exceeding your expectations.
Experience the Inotiv Difference
Our Program Management team is here to help you navigate your development process—clarifying potential risks and benefits and connecting you with the right subject matter experts when needed. Contact us today to learn more about how we can support your goals.
Program Management (PM) Frequently Asked Questions (FAQs)
What is the role of a Program Manager at Inotiv?
An Inotiv, Program Manager is your dedicated point of contact, overseeing all aspects of your project from initiation to completion. They ensure timely delivery, manage communication, and coordinate resources across different departments to guarantee project success.
Who is eligible for Program Management services?
To be eligible for our PM services, clients must be engaged in multiple, related projects that contribute to a shared goal e.g., Investigational New Drug (IND) Application, Premarket Approval (PMA), etc. This often involves a complex program that include numerous in-life studies supported by multiple Study Directors with supporting chemistry and pathology requirements.
How does Inotiv select a Program Manager for my project?
Inotiv carefully assesses your project's specific needs and assigns a Program Manager with the appropriate expertise and experience.
Can I request a specific Program Manager?
While we strive to accommodate your requests, the best Program Manager for your project is often determined by their expertise and availability.
How often will I communicate with my Program Manager?
The frequency of communication depends on your project's complexity and your preferences. However, you can expect regular updates and open communication throughout the project lifecycle.
A typical communication plan includes:
- Type of preferred communication (email, teleconference, video calls, etc.)
- Timing and frequency of standing conference calls
- A list of participants involved in the communication
- Defined content to be covered in each conference call
What if I have a change order or need to modify the project scope?
Your Program Manager will work closely with you to assess the impact of any changes and develop a revised project plan accordingly.
What should I expect from Inotiv’s Program Management Team?
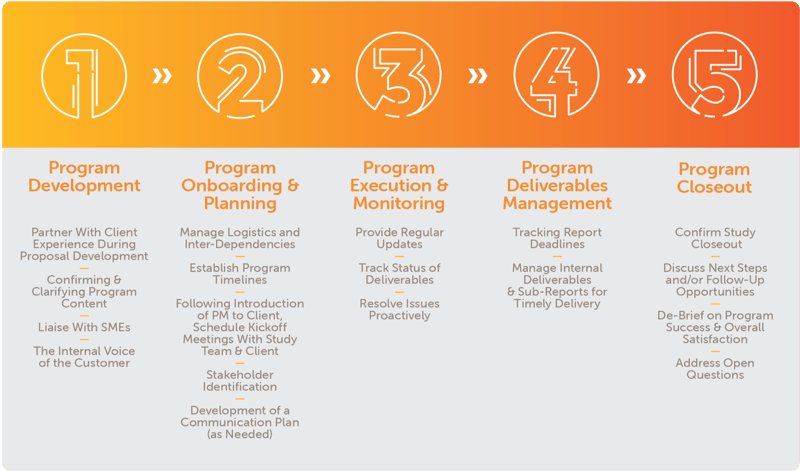
Inotiv’s Program Management team will evaluate your upcoming program needs and assign a dedicated program manager to guide you through the process.
Our proven process follows the following steps:
What other services does Inotiv Offer?
Inotiv provides a comprehensive range of services including:
- Disease Pharmacology
- Drug Metabolism and Pharmacokinetics (DMPK)
- Discovery Bioanalysis and Biomarkers
- Cell and Molecular Biology
- Predictive and Computational Toxicology
- Toxicogenomics
- Targeted Proteomics
- Histology
- Pathology
- Toxicology
- Exploratory Toxicology
- Genetic Toxicology
- Safety Pharmacology
- Development and Reproductive Toxicology (DART)
- Carcinogenicity
- Bioanalysis for Large Molecule
- Bioanalysis for Small Molecule
- Archiving Solutions
- Surgical Support and Medical Device Testing
- Medical Device Pathology
- Research Models and Services
- Teklad™ Diet, Bedding and Enrichment
- SEND (Standard for Exchange of Nonclinical Data)
- Consultative Drug Discovery Programs
Use our deep expertise in safety assessment to your advantage. At Inotiv, our team understands the importance and complexity of the drug development process and will be there to guide you to success by offering an integrated safety pharmacology solution as a standalone safety pharmacology service or as part of a full IND/CTA program.

