Rheumatoid Arthritis Models
Comprehensive Solutions for Rheumatoid Arthritis Drug Discovery and Development

Rheumatoid arthritis is a chronic autoimmune disease marked by systemic inflammation, primarily targeting synovial joints. The persistent inflammatory response within the synovium can progressively degrade articular cartilage and subchondral bone, potentially leading to joint destruction, reduced mobility, and eventual ankylosis. Effective disease pharmacology is crucial, as many patients remain unresponsive to current treatments.
As a preclinical CRO, Inotiv advances drug discovery for this disease by offering validated rheumatoid arthritis models and conducting pharmacology studies to assess therapeutic efficacy and safety in relevant animal systems.
Rheumatoid Arthritis In Vivo Models
Rheumatoid arthritis in vivo models are essential tools in preclinical drug discovery, enabling the study of immune system involvement and disease mechanisms in autoimmune arthritis. With multiple induction methods available, these models reliably replicate key features of rheumatoid arthritis — including joint inflammation, bone erosion, and cartilage degradation—supporting the development and evaluation of new therapies. By providing a controlled environment to study these factors, arthritis models are invaluable in advancing our understanding and treatment of rheumatoid arthritis.
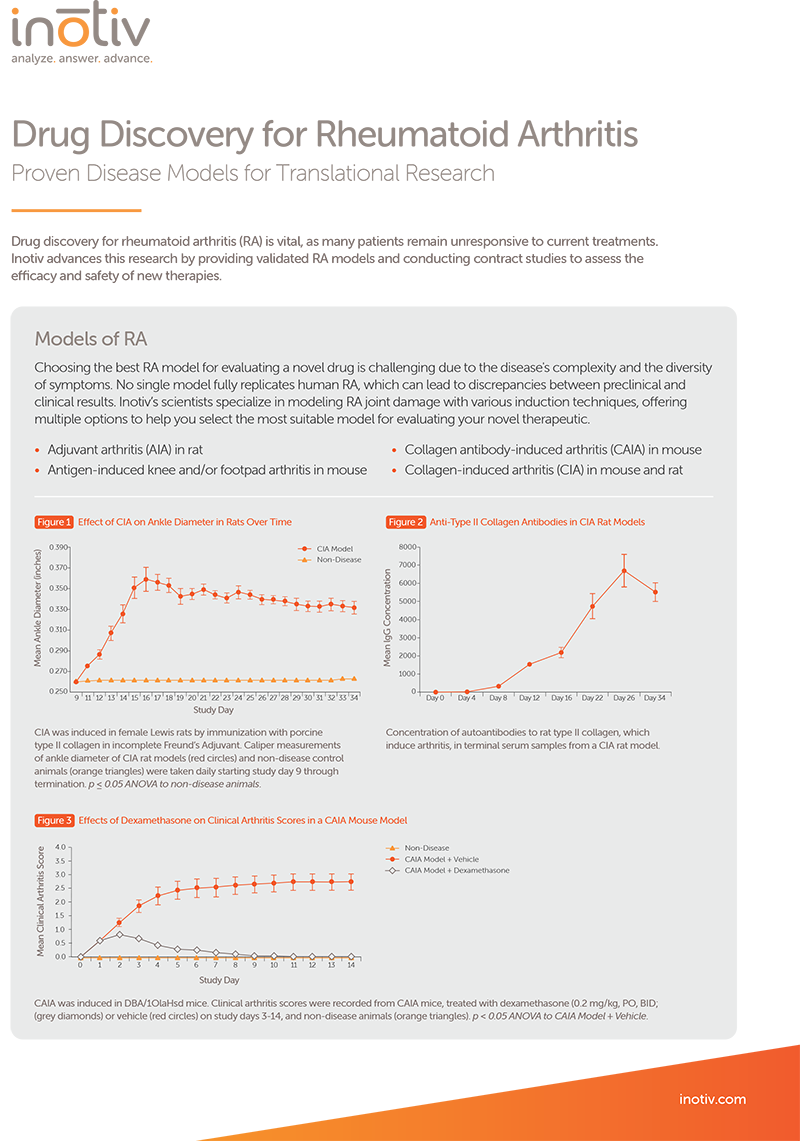
Developing effective animal models for autoimmune diseases like rheumatoid arthritis is complex due to the diverse symptoms and various methodologies required for modeling. Inotiv’s validated models replicate key features of human rheumatoid arthritis pathology—including synovial inflammation, pannus formation, and joint erosion—and serve as a reliable platform for in vivo pharmacology studies to assess the efficacy and safety of new therapies.
Collagen-Induced Arthritis Model
Model Summary
Collagen-induced arthritis (CIA) is induced in susceptible strains of mice and rats by immunizing them with type II collagen, a key component of articular cartilage. This process triggers an autoimmune response involving collagen-specific T cell activation and antibody production against both the immunogen and autoantigen. The resulting polyarthritis exhibits clinical and pathological features similar to rheumatoid arthritis, such as synovitis, cartilage erosion, and bone damage. Susceptibility to developing polyarthritis is linked to MHC class II molecules.
CIA is a widely used model for in vivo pharmacology studies, providing valuable insights into rheumatoid arthritis mechanisms and the efficacy of potential therapies. When used in preclinical research, the CIA model aids in the development of anti-arthritic treatments by assessing both the progression of the disease and therapeutic interventions.
Example Data

CIA rat models were treated on Days 11-16 with vehicle, an anti-IL-17A antibody, or dexamethasone. (A) Caliper measurements of ankle diameter over time for CIA rat models treated with vehicle (dark grey squares), an anti-IL-17A antibody (orange triangles), or dexamethasone (red circles) . Non-disease controls (light grey squares) were included for comparison. *p < 0.05 Disease + Vehicle vs No Disease. ϯ p < 0.05 to Disease + Vehicle. (B) Mean histopathology scores for inflammation (dark grey bars), pannus formation (yellow bars), cartilage damage (light grey bars), bone resorption (red bars) and periosteal bone formation (orange bars) in the ankle. *p < 0.05 Disease + Vehicle vs No Disease. ϯ p < 0.05 to Disease + Vehicle.

Clinical arthritis scores were recorded from CIA mouse models that were treated on study days 2-11 with dexamethasone (red circles), enbrel (orange triangles), or vehicle (dark grey boxes), and non-disease controls (light grey boxes).
References
- Trentham, D.E. et al. (1977) J Exp Med 146: 857–868.
- Geiger, T. et al. (1993) Clin Exp Rheumatol 11:515-522.
- Wooley, P.H. et al. (1993) Arthritis Rheum 36:1305-1314.
- Joosten, L.A.B. et al. (1994) Agents Actions 41:C174-C176.
- Terato, K. et al. (1995) Autoimmunity 22: 137–147.
- Joosten, L.A.B. et al. (1996) Arthritis Rheum 39:797-809.
- Bakker, A.C. et al. (1997) Arthritis Rheum 40:893-900.
- Bendele, A. M. (2001) J Musculoskel Neuronal Interact 4: 363– 376.
- Nandakumar, K.S. et al. (2003) Am J Pathol 163: 1827–1837.
- Scarneo, S.A. et al. (2022) Sci Rep 12:18091.
Collagen Antibody-Induced Arthritis Model
Model Summary
The collagen antibody-induced arthritis (CAIA) model is a commonly used rodent model of rheumatoid arthritis. It is produced by injecting an antibody cocktail targeting type II collagen into mice. This approach rapidly induces polyarthritis, leading to inflammation, synovitis, and joint damage like rheumatoid arthritis and the CIA model. However, unlike the CIA model, which requires active immunization and a functional immune system, CAIA uses passive transfer of type II collagen-specific antibodies, bypassing MHC class II involvement. Additionally, the CAIA model's rapid onset and reproducibility make it valuable in drug development for quickly assessing in vivo efficacy of anti-inflammatory treatments. It is preferred when focusing on acute inflammation, screening therapies efficiently, or working with altered immune responses, offering a versatile and efficient platform for in vivo preclinical rheumatoid arthritis research.
Example Data

Clinical arthritis scores were recorded from CAIA mice, treated with dexamethasone (grey diamonds) or vehicle (red circles), and non-disease animals (orange triangles) on study days 3-14. p < 0.05 ANOVA to CAIA Model + Vehicle.

(A) Mean histopathology scores for inflammation (dark grey bars), pannus formation (yellow bars), cartilage damage (light grey bars), bone resorption (red bars) and periosteal bone formation (orange bars) in the fore paw of CAIA mouse models, treated with dexamethasone or vehicle, and non-disease animals. *p < 0.05 to CAIA Model + Vehicle. (B) Representative photomicrographs of a fore paw from a CAIA mouse model stained with toluidine blue. Histology reveals inflammation (*), cartilage damage (arrow), periosteal bone formation (P), bone resorption (black arrowhead) and pannus formation (open arrowhead).
References
Adjuvant Arthritis Model
Model Summary
The adjuvant arthritis (AIA) rat model is a widely used preclinical tool for evaluating anti-inflammatory and immunomodulatory therapies. Arthritis is induced in rats by injecting Freund’s complete adjuvant (FCA) at the base of the tail, triggering a systemic autoimmune response characterized by polyarticular inflammation, bone resorption, periosteal proliferation, and mild cartilage destruction. Clinical signs of arthritis typically appear around days 11-13 post-induction. While its lesions are less analogous to human rheumatoid arthritis than those of the CIA model, the AIA model is favored for its reliability, cost-effectiveness, and extensive comparative data.
In addition to joint pathology, the AIA model also exhibits systemic effects such as splenomegaly, hepatomegaly, and anterior uveitis, making it valuable for assessing both joint-specific and systemic therapeutic impacts in in vivo pharmacology.
Example Data

AIA rat models were treated on Days 7-23 with vehicle (dark grey squares), baricitinib (orange triangles), or tofacitinib (red circles). Non-disease controls (light grey squares) were included for comparison. (A) Caliper measurements of ankle diameter over time. (B) Average body weight over time.
References
- Bendele, A. M. (2001) J Musculoskel Neuronal Interact 4: 363– 376.
- Pearson C.M. (1956) Proc Soc Exp Biol Med 91:95-101.
- Carlson R.P. et al. (1985) Int J Immunopharmacol 7:811-826.
- Benslay D.N. and Bendele, A.M. (1991) Agents Actions 34:254-256.
- Chang, Y.H. et al. (1980) Arthritis Rheum 23:62-71.
- Bendele, A. et al. (1999) Arthritis Rheum 42:498-506.
- Bendele, A. et al. (1999) J Rheumatol 26:1225-1229.
- McComb, J. et al. (1999) J Rheumatol 26:1347-1351.
- Bendele, A.M. et al. (1999) Inflamm Res 48:453-460.
- Bendele, A.M. et al. (1999) Clin Exp Rheumatol 17:553-560.
- Bendele, A.M. et al. (2000) Arthritis Rheum 43:2648-2659.
Antigen-Induced Knee/Footpad Arthritis Model
Model Summary
The antigen-induced arthritis model is a T-cell-dependent animal model used to study rheumatoid arthritis. Animals are first immunized with an antigen, typically methylated bovine serum albumin (mBSA), mixed with an adjuvant like Freund’s complete adjuvant to elicit an immune response. After a sensitization period, the antigen is injected directly into the knee joint or footpad, leading to acute inflammation and progressive joint destruction. This model is versatile, applicable across species, and closely mimics the immune-driven pathogenesis of human rheumatoid arthritis, making it valuable for arthritis research.
References
- Bendele, A. M. (2001) J Musculoskel Neuronal Interact 4: 363– 376.
- Brackertz, D. et al. (1977) Arthritis Rheum 20:841-850.
- Hunneyball, I.M. et al. (1986) Agents Actions 18:384-393.
- van de Loo, F.A. et al. (1992) J Rheumatol 19:348-356.
- van de Loo, A.A. et al. (1995) Amer J Pathol 146:239-249.
- van de Loo, F.A. et al. (1995) Arthritis Rheum 38:164-172.
- van Meurs, J.B. et al. (1998) Arthritis Rheum 41:647-656.
Strep Cell Wall Reactivation Arthritis Model
Model Summary
The strep cell wall (SCW) reactivation arthritis model is a reproducible system for studying arthritis, using peptidoglycan-polysaccharide (PGPS) components from streptococcal cell walls to induce joint inflammation. Arthritis is initiated by injecting SCW fragments directly into the joint or systemically, triggering acute inflammation. IV (reactivation) doses can then be given at specific intervals (2-6 weeks apart) that results in a flare of the arthritis, which persists for 5-7 days. This model allows researchers to study the mechanisms of disease progression and evaluate therapeutic interventions, particularly in chronic and relapsing forms of arthritis. The model also provides the ability to study the effects of locally delivered test articles (intra-articular) in the context of a mono-arthritis, allowing for the ability to look at behavioral measurements like weight bearing and gait changes associated with pain.
SKG Transgenic Mouse Model
Model Summary
The SKG mouse is a widely used transgenic model for studying rheumatoid arthritis, as it develops chronic, progressive arthritis that closely resembles human disease. This phenotype arises from a point mutation in the ZAP-70 gene, which leads to defective T cell receptor signaling. As a result, thymic negative selection is impaired, allowing self-reactive T cells to escape into the periphery. SKG mice spontaneously develop arthritis under specific pathogen-free (SPF) conditions, though disease onset and severity can be accelerated by exposure to microbial or fungal components such as zymosan. The model is characterized by synovial inflammation, pannus formation, and bone and cartilage destruction, making it particularly valuable for investigating autoimmune mechanisms, environmental triggers of disease, and potential therapeutic interventions for rheumatoid arthritis.
Example Data

Clinical arthritis scores were recorded from SKG transgenic mice that were treated with a JAK inhibitor (orange triangles) or vehicle (red circles) on study days 1-30. The animals received a single dose of zymosan (4 mg/mL) at the beginning of the study.
TNF-α TRANSGENIC MOUSE MODEL
Model Summary
The TNF-α transgenic mouse is a well-established model for rheumatoid arthritis, as it develops spontaneous, chronic, and progressive polyarthritis driven by constitutive expression of human TNF-α. In rheumatoid arthritis, excess TNF-α stimulates synovial fibroblasts and other resident cells, leading to increased production of proteolytic enzymes such as matrix metalloproteinases and cathepsins. This heightened enzymatic activity promotes breakdown of collagen and proteoglycans, ultimately driving cartilage degradation, bone resorption, and joint erosion. TNF-α transgenic mice display disease hallmarks including synovial hyperplasia, inflammatory cell infiltration, pannus formation, and progressive cartilage and bone destruction. Importantly, arthritis in this model develops independently of adaptive immune activation, making it a valuable system for studying TNF-α–mediated inflammatory mechanisms and testing the efficacy of anti-TNF therapies and other disease-modifying agents.
Example Data

Mean histopathology scores for inflammation (dark grey bars), pannus formation (yellow bars), cartilage damage (light grey bars), bone resorption (red bars) and periosteal bone formation (orange bars) in the ankles of TNF-α transgenic mice, treated with TNF-α blocker or vehicle, and non-disease animals. *p < 0.05 TNF-α Blocker vs Vehicle. ϯp < 0.05 Vehicle vs Non-Disease.
Not seeing the model you need? Contact us to discuss developing a new rheumatoid arthritis model for your drug development program.
Clinical Assessment of Rheumatoid Arthritis Models
Arthritis assessments in rat and mouse rheumatoid arthritis models utilize diverse methods to evaluate disease progression and therapeutic outcomes. A key approach involves clinical scoring systems that grade joint swelling and redness on a standardized scale. These subjective evaluations are often paired with objective measurements, such as caliper-based joint thickness or paw volume analysis, to provide a comprehensive assessment in in vivo pharmacology studies.
Histopathological Assessment of Rheumatoid Arthritis Models
Histopathology is a vital component of rheumatoid arthritis animal models, offering detailed insights into tissue-level changes that occur during disease progression and treatment. Inotiv’s ACVP board-certified pathologists apply advanced histology and pathology techniques to assess critical features such as inflammation, pannus formation, cartilage damage, bone resorption, periarticular matrix deposition, and periosteal new bone formation—empowering confident decision-making in therapeutic development.
To support these evaluations, joints including ankles, knees, and paws are carefully sectioned, embedded in paraffin blocks, and stained with toluidine blue to reveal structural changes. Each tissue section is scored for relevant pathological features, providing a comprehensive view of both acute and chronic phases of arthritis and the impact of investigational therapies.
Learn more about our histology services.
Customize Your Rheumatoid Arthritis Drug Discovery Study
Our research services are tailored to advance drug discovery for rheumatoid arthritis through detailed evaluations. We offer comprehensive analyses of joint histopathology, biomarkers of inflammation, bone remodeling, and immune system modulation in well-validated in vivo models. These services can be customized to assess the in vivo efficacy and safety of novel treatments, supporting the development of therapies for rheumatoid arthritis.
When paired with in vitro and ex vivo pharmacology services, our flexible approach ensures an integrated strategy for evaluating potential therapies. Some optional study endpoints include:
- PK/PD blood collections
- Cytokine/chemokine analysis with ELISA and Luminex® assays
- CBC/clinical chemistry analysis
- Soft tissue collection
- Immunohistochemistry analysis
These are just a few examples of the assays we can perform. Contact us to learn more about how we can customize studies to meet your specific research needs.
Pharmacokinetics
(DMPK) Drug Metabolism and
Pharmacokinetics
(DMPK) Whatever you need, from method development to metabolite ID, our phase-appropriate bioanalytical services help you analyze, answer, and advance.
Our industry-leading histology laboratories support your discovery and nonclinical development projects with a wide range of services. From tissue processing to digital analysis, our team delivers the quality you expect.
We offer customized end-to-end bioanalytical solutions to achieve your milestones effectively and on schedule, supporting the development of a wide range of therapeutics from discovery through Phase IV.